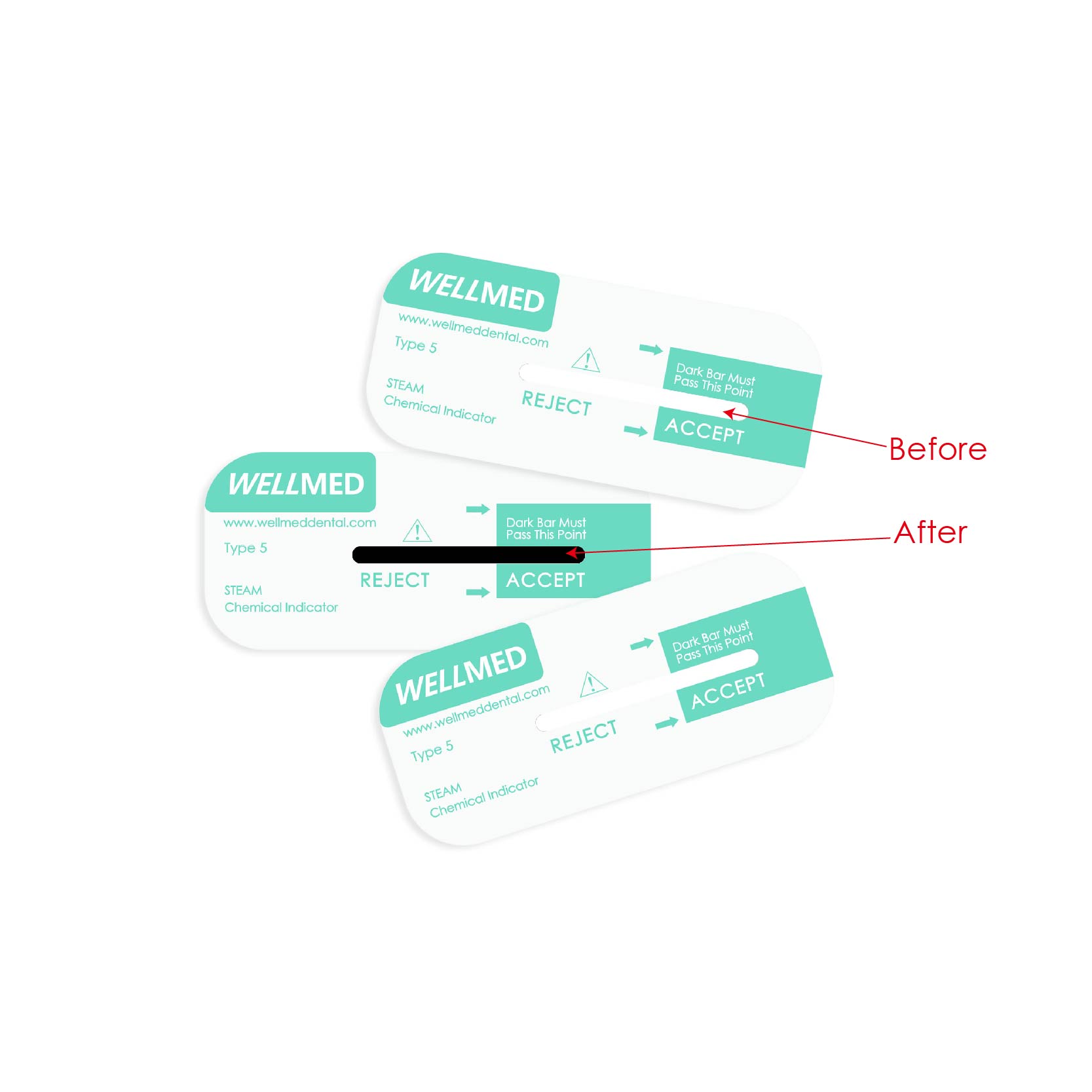
Quantity:
Bag of 500
Steam Sterilization Integrators
Description:
- Ability to provide a distinct pass-or-fail result, providing confidence to safely release the instruments in every steam sterilization cycle;
- Detects potential sterilizer failures which can result from incorrect packaging, overloading or malfunctions of the sterilizer;
- Used as an additional monitoring tool for the release of sterilized loads versus quarantined loads until a biological indicator test result is reported as negative;
- Used daily, or with every load, is a means of improving patient safety and reduces the cost and disruption of potential recalls when a biological indicator test fails;
- Parallels the spore death curve in a normal steam sterilization range with a margin of safety.
| Items | Description |
| #SSI-001 | Type 5, Green |
Steam Sterilization Integrators Manufacturer:
- Extensive Expertise as a Steam Sterilization Integrators Manufacturer: With years of experience as a steam sterilization integrators manufacturer, we have developed a deep understanding of the industry. As a reputable manufacturer, we excel in producing high-quality integrators that meet the precise requirements of professionals and users. Our expertise in this field ensures superior performance and maximum effectiveness, making us a trusted choice for various applications worldwide.
- Continuous Innovation as a Leading Steam Sterilization Integrators Manufacturer: As a prominent manufacturer, we prioritize continuous innovation in our manufacturing processes. Our dedicated team of experts, with their extensive knowledge and experience, constantly seeks to enhance our products. By integrating advanced features and leveraging cutting-edge technologies, we ensure that our integrators stand out in the market. Our commitment to innovation allows us to provide customers with state-of-the-art solutions that meet the evolving needs of the industry, setting us apart as a leader in the field.
SIMILAR Products
Sanax Protective Products
Leading Manufacturer of Dental & Industrial Safety Disposable Products
Founded in 1990, Sanax Protective Products has strived to manufacture the best possible infection control and disposable products to our valued customers worldwide. Our products are sold in the medical, dental, veterinary and tattoo markets. We have partnered with various manufacturers in China, Thailand and worldwide to produce products that meet or exceed governmental requirements in the U.S., Europe, Australia, Japan and elsewhere.
Together with our dental product manufacturer partners, we work closely with NIOSH (National Institute for Occupational Safety and Health) and the FDA (Food and Drug Administration) to insure all our products are up-to-date from a regulatory standpoint. All our factories are ISO certified.